- Synthetic anti-infective drugs
- Medications for the digestive system
- Antipyretic and analgesic drugs
- Medications for the blood system
- Medications for the respiratory system
- Anti-allergic drugs
- Medications for the urinary system
- Diagnostic medications
- Immunosuppressive and immunomodulatory drugs
- Vitamins and mineral supplements
- Antioxidants and medications for osteoporosis
- Antiparasitic drugs
- Ophthalmic medications
- Amino acids and their derivatives
- Dermatological medications
- Medications for the circulatory system
- Antitumor drugs
- Medications for the nervous system
- Hormonal and endocrine function-regulating drugs
- Antibiotics
- Others
CAS NO.: 274693-27-5
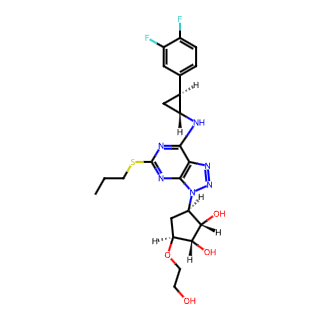



Ticagrelor (trade names: Brilinta in the US, Brilique in Europe) is an oral antiplatelet medication primarily used in the prevention and treatment of cardiovascular diseases.
Basic Information
Generic Name: Ticagrelor
Chinese Name: Tigrelor, Ticagrelor
Drug Type: Prescription-only medication
Chemical Name: (1S,2S,3R,5S)-3-[7-{[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5-(propylsulfanyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethyl)cyclopentane-1,2-diol
Indications
Ticagrelor is indicated for the treatment of patients with acute coronary syndrome (ACS), including those with unstable angina, non-ST-segment elevation myocardial infarction (NSTEMI), or ST-segment elevation myocardial infarction (STEMI), as well as patients undergoing medical therapy or percutaneous coronary intervention (PCI). It significantly reduces the incidence of thrombotic cardiovascular events such as cardiovascular death, myocardial infarction, or stroke.
Precautions
Bleeding Risk: Ticagrelor may cause significant bleeding, including skin, nasal, gingival, and gastrointestinal bleeding. Severe cases may lead to visceral bleeding. Therefore, it is contraindicated in patients with active pathological bleeding or a history of intracranial bleeding.
Drug Interactions: This medication should be used in conjunction with aspirin, with aspirin maintenance doses ranging from 75 to 100 mg daily. Aspirin maintenance doses greater than 100 mg may reduce the clinical efficacy of Ticagrelor in reducing composite endpoint events.
Monitoring: During treatment with Ticagrelor, patients should be regularly monitored for coagulation function and bleeding, and medical attention should be sought immediately if abnormal bleeding symptoms occur.
Special Populations: The safety and effectiveness of Ticagrelor have not been established or are contraindicated in patients under 18 years old, those with moderate-to-severe hepatic impairment, and those planning to undergo emergency coronary artery bypass grafting (CABG).
Adverse Reactions
Common adverse reactions to Ticagrelor include dyspnea, contusions, epistaxis, gastrointestinal bleeding, hematuria, bleeding at vascular puncture sites, etc. It may also cause bradycardia, gout flares, and liver function abnormalities. In rare cases, it may lead to severe allergic reactions such as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat.
Drug Interactions
Ticagrelor interacts with multiple medications, including anticoagulant drugs such as aspirin, clopidogrel, heparin, and nonsteroidal anti-inflammatory drugs (NSAIDs). Therefore, during treatment with Ticagrelor, patients should avoid taking other medications, especially those that may increase the risk of bleeding, without consulting a physician.
Conclusion
Ticagrelor is an effective antiplatelet medication that plays a crucial role in the treatment of patients with acute coronary syndrome. However, its use must strictly follow medical advice and precautions to ensure efficacy and safety. If any discomfort or abnormal symptoms occur during treatment, patients should seek medical attention promptly and inform their physicians of their use of Ticagrelor.

Tai Yau Street, San Po Kong, Kowloon, Hong Kong, China.