- Synthetic anti-infective drugs
- Medications for the digestive system
- Antipyretic and analgesic drugs
- Medications for the blood system
- Medications for the respiratory system
- Anti-allergic drugs
- Medications for the urinary system
- Diagnostic medications
- Immunosuppressive and immunomodulatory drugs
- Vitamins and mineral supplements
- Antioxidants and medications for osteoporosis
- Antiparasitic drugs
- Ophthalmic medications
- Amino acids and their derivatives
- Dermatological medications
- Medications for the circulatory system
- Antitumor drugs
- Medications for the nervous system
- Hormonal and endocrine function-regulating drugs
- Antibiotics
- Others
CAS NO.: 129-06-6
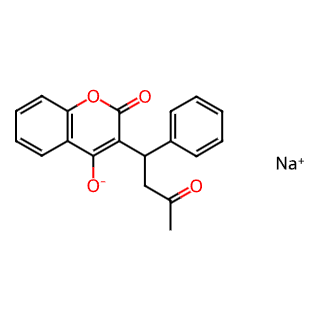



Warfarin Sodium
Warfarin Sodium, also known as Coumadin, is an organic compound primarily used as an anticoagulant medication. Below is a detailed introduction to Warfarin Sodium:
Basic Information
English Name: Warfarin Sodium
Chinese Name: 华法林钠
Chemical Formula: C19H15NaO4
Molecular Weight: 330.310
CAS Registry Number: 129-06-6
EINECS Number: 204-929-4
Appearance: White to pale yellow powder
Physical and Chemical Properties
Density: 1.307 kg/m³
Boiling Point: 515.2 °C
Flash Point: 188.8 °C
Pharmacological Action
Warfarin Sodium is a vitamin K epoxide reductase inhibitor that exerts its anticoagulant effect by antagonizing the action of vitamin K, thereby inhibiting the synthesis of coagulation factors. It is primarily used for the prevention and treatment of thromboembolic disorders such as thrombophlebitis, deep vein thrombosis, pulmonary embolism, and as an adjunctive therapy in myocardial infarction, including the treatment of atrial fibrillation with pulmonary embolism and as an adjunctive therapy in coronary occlusion.
Adverse Reactions
Bleeding: The primary adverse reaction is bleeding, ranging from mild local ecchymosis to major hemorrhage. The most common is nasal bleeding, followed by bleeding from the gums, gastrointestinal tract, genitourinary system, spinal cord, brain, pericardium, lungs, adrenal glands, or liver.
Other Adverse Reactions: Occasional nausea, vomiting, diarrhea, leukopenia, granulocytosis, nephrosis, pruritic rash, and allergic reactions.
Specific Adverse Reactions: Patients receiving this medication occasionally experience (fatal) gangrene, embolic cyanosis of the skin, subcutaneous tissue, or other tissues, purple toenail syndrome (occurring 3-8 weeks after treatment), vasculitis, and local thrombosis. Ninety percent of these cases involve women, typically occurring 2-10 days after starting treatment.
Contraindications
Hypersensitivity to Warfarin Sodium or any of its excipients.
Severe bleeding tendency, recent intracranial hemorrhage, or a tendency toward intracranial hemorrhage.
Within 3 days after surgery, patients with a bleeding tendency (e.g., hemophilia, thrombocytopenic purpura), severe liver or kidney disease, active peptic ulcer, or before brain, spinal cord, or ophthalmic surgery.
Pregnancy.
Precautions
During long-term use at the lowest maintenance dose, if surgery is required, intravenous vitamin K1 injection 50 mg can be administered first, but discontinuation is necessary before central nervous system or ophthalmic surgery.
Monitor PT+A during treatment, paying attention to any concurrent medications that may affect the efficacy of Warfarin Sodium to adjust the dosage accordingly.
Avoid concomitant use with medications that enhance or diminish the anticoagulant effect of Warfarin Sodium, such as aspirin and sodium salicylate (enhancing) or phenytoin and barbiturates (diminishing).
In summary, Warfarin Sodium is an essential anticoagulant medication, but its use requires strict adherence to medical advice, attention to dosage, and monitoring of coagulation function to prevent adverse reactions.

Tai Yau Street, San Po Kong, Kowloon, Hong Kong, China.