- Synthetic anti-infective drugs
- Medications for the digestive system
- Antipyretic and analgesic drugs
- Medications for the blood system
- Medications for the respiratory system
- Anti-allergic drugs
- Medications for the urinary system
- Diagnostic medications
- Immunosuppressive and immunomodulatory drugs
- Vitamins and mineral supplements
- Antioxidants and medications for osteoporosis
- Antiparasitic drugs
- Ophthalmic medications
- Amino acids and their derivatives
- Dermatological medications
- Medications for the circulatory system
- Antitumor drugs
- Medications for the nervous system
- Hormonal and endocrine function-regulating drugs
- Antibiotics
- Others
CAS Number: 69-57-8



I. Basic Information
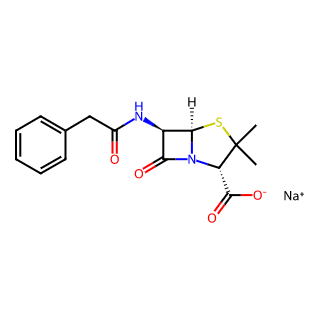
Product Name: Penicillin G Sodium
CAS Number: 69-57-8
Molecular Formula: C16H17N2NaO4S (also can be expressed as C16H18N2O4S·Na, with slight differences in notation but essentially the same)
Molecular Weight: 356.372
II. Physical Properties
Appearance: White to grayish-white crystalline powder, odorless or with a slight specific odor.
Melting Point: 209~212°C (some sources indicate a decomposition point of 215°C)
Density: 1.41
Boiling Point: 663.3°C at 760 mmHg
Refractive Index: 300° (C=2, in water)
Specific Rotation: [α]D24.8 +301° (2% aqueous solution)
Solubility: Extremely soluble in water, soluble in ethanol, insoluble in fatty oils and liquid paraffin. The solubility in water is approximately 5~10g/100mL (at 25°C).
III. Chemical Properties
Stability: The crystalline form of penicillin G sodium is relatively stable, but its aqueous solution is prone to decomposition at room temperature and cannot be sterilized by boiling. Penicillin G sodium is hygroscopic and can be inactivated by acids, bases, oxidants, penicillinases, etc., which open the β-lactam ring of penicillin.
IV. Uses and Pharmacological Effects
Uses: Penicillin G sodium is primarily used for infections caused by sensitive bacteria such as Streptococcus, Pneumococcus, and Meningococcus.
Pharmacological Effects: It exerts bactericidal effects by interfering with the formation of bacterial cell walls. It has antibacterial activity against G+ cocci and G+ bacilli (such as diphtheria, tetanus, and anthrax bacilli), spirochetes, clostridia, actinomycetes, and some anaerobic bacilli.

Tai Yau Street, San Po Kong, Kowloon, Hong Kong, China.