- Synthetic anti-infective drugs
- Medications for the digestive system
- Antipyretic and analgesic drugs
- Medications for the blood system
- Medications for the respiratory system
- Anti-allergic drugs
- Medications for the urinary system
- Diagnostic medications
- Immunosuppressive and immunomodulatory drugs
- Vitamins and mineral supplements
- Antioxidants and medications for osteoporosis
- Antiparasitic drugs
- Ophthalmic medications
- Amino acids and their derivatives
- Dermatological medications
- Medications for the circulatory system
- Antitumor drugs
- Medications for the nervous system
- Hormonal and endocrine function-regulating drugs
- Antibiotics
- Others
CAS NO.: 147403-03-0



Basic Information
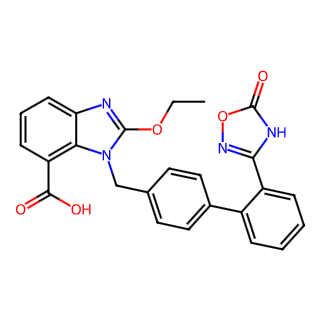
Chinese Name: Azilsartan
English Name: Azilsartan
Synonyms: 2-Ethoxy-1-[[2'-(4,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl]benzimidazole-7-carboxylic acid
CAS Number: 147403-03-0
Molecular Formula: C25H20N4O5
Molecular Weight: 456.45 (Note: slight variations may exist in different sources but are generally close)
Physical and Chemical Properties
Melting Point: 188°C (decomposes)
Density: 1.42
Solubility: Soluble in DMSO at 15 mg/mL (clear solution), slightly soluble in methanol, very slightly soluble in ethanol
Uses
Azilsartan is a drug under development for the treatment of hypertension as an angiotensin II receptor antagonist. It is currently the only angiotensin II receptor antagonist (sartan class) drug in late-stage clinical development. It was approved for marketing in Japan on January 18, 2012, and has obtained marketing authorization in the United States and the European Union.
Safety and Storage
Hazard Description: Specific acute toxicity data is not provided, but it should be considered a potentially hazardous substance, and chemical safety operating procedures should be followed.
Storage Conditions: Should be stored under refrigerated conditions at 2-8°C, away from incompatible materials such as oxidizers, and kept in tightly closed containers.
Precautions
When handling azilsartan, wear appropriate personal protective equipment such as protective gloves, safety goggles, and dust masks.
Avoid direct contact with skin, eyes, and clothing. If contact occurs, immediately flush with copious amounts of water and seek medical attention.
Comply with relevant chemical safety management and use regulations.

Tai Yau Street, San Po Kong, Kowloon, Hong Kong, China.