- Synthetic anti-infective drugs
- Medications for the digestive system
- Antipyretic and analgesic drugs
- Medications for the blood system
- Medications for the respiratory system
- Anti-allergic drugs
- Medications for the urinary system
- Diagnostic medications
- Immunosuppressive and immunomodulatory drugs
- Vitamins and mineral supplements
- Antioxidants and medications for osteoporosis
- Antiparasitic drugs
- Ophthalmic medications
- Amino acids and their derivatives
- Dermatological medications
- Medications for the circulatory system
- Antitumor drugs
- Medications for the nervous system
- Hormonal and endocrine function-regulating drugs
- Antibiotics
- Others
CAS NO.: 148849-67-6
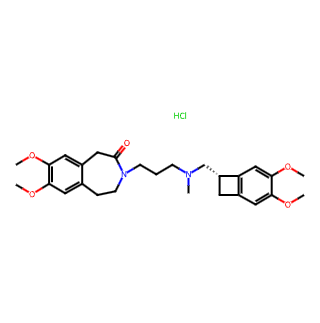



Ivabradine Hydrochloride
Basic Information:
Generic Name: Ivabradine Hydrochloride
Chemical Name: 3-(3-{[((7S)-3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methyl]methylamino}propyl)-1,3,4,5-tetrahydro-7,8-dimethoxy-2H-3-benzazepin-2-one hydrochloride
Molecular Formula: C27H36N2O5•HCl
Molecular Weight: 505.1 (may vary slightly depending on sources, e.g., 505.05)
CAS Number: 148849-67-6
Indications:
Indications: Ivabradine Hydrochloride is indicated for the treatment of patients with chronic heart failure (NYHA class II-IV) with systolic dysfunction and a sinus rhythm with a heart rate ≥75 beats per minute (bpm), in conjunction with standard therapy (including beta-blockers) or when beta-blockers are contraindicated or not tolerated.
Adverse Reactions:
The most common adverse reactions reported in clinical studies for Ivabradine Hydrochloride include phosphenes (visual brightness) and bradycardia, both of which are dose-dependent. Other more common adverse reactions include dizziness, headache, blurred vision, cardiac disorders (such as first-degree atrioventricular block, ventricular extrasystoles, atrial fibrillation), etc.
Less common or rare adverse reactions include eosinophilia, hyperuricemia, syncope, diplopia, visual disturbances, palpitations, hypotension, dyspnea, nausea, vomiting, diarrhea, angioedema, rash, muscle spasms, increased serum creatinine levels, etc.
Storage Conditions:
Ivabradine Hydrochloride should be stored in a dry, cool, well-ventilated place, protected from direct sunlight and excessive heat. Specific storage conditions may vary depending on different manufacturers and packaging specifications.

Tai Yau Street, San Po Kong, Kowloon, Hong Kong, China.