- Synthetic anti-infective drugs
- Medications for the digestive system
- Antipyretic and analgesic drugs
- Medications for the blood system
- Medications for the respiratory system
- Anti-allergic drugs
- Medications for the urinary system
- Diagnostic medications
- Immunosuppressive and immunomodulatory drugs
- Vitamins and mineral supplements
- Antioxidants and medications for osteoporosis
- Antiparasitic drugs
- Ophthalmic medications
- Amino acids and their derivatives
- Dermatological medications
- Medications for the circulatory system
- Antitumor drugs
- Medications for the nervous system
- Hormonal and endocrine function-regulating drugs
- Antibiotics
- Others
CAS Number: 159351-69-6
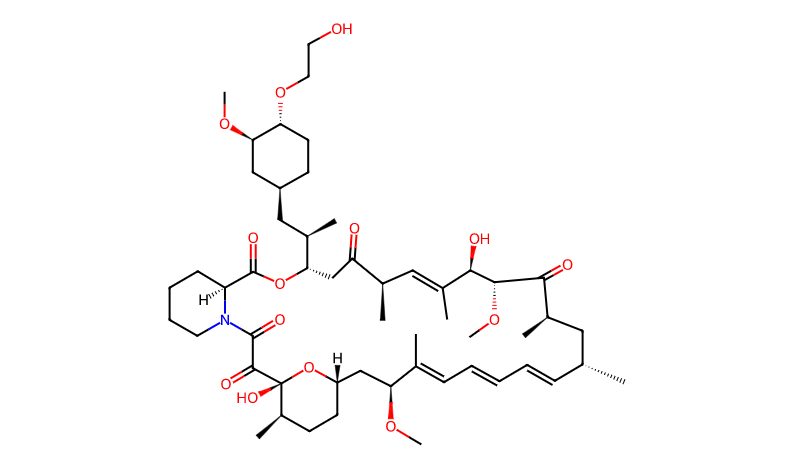



I. Basic Information
Product Name: Everolimus
CAS Number: 159351-69-6
Molecular Formula: C53H83NO14
Molecular Weight: 958.224 (alternatively, 957.581 or 958.22400, depending on measurement precision)
II. Physical Properties
Appearance and Characteristics: Grayish-white to yellow powder
Density: 1.18g/cm³ (predicted value, with slight fluctuations possible, such as 1.18±0.1g/cm³)
Boiling Point: 998.7ºC (predicted value, subject to variations due to multiple factors)
Flash Point: 557.8ºC or 2ºC (the latter may be a predicted value or a special value under experimental conditions)
Refractive Index: 1.548
Storage Conditions: Typically stored at -20ºC to ensure drug stability and effectiveness
III. Chemical Properties
Type: A kinase drug that interferes with cell communication to prevent tumor cell growth, specifically an oral mammalian target of rapamycin (mTOR) inhibitor
Inhibitory Activity: Forms an inhibitory complex mTORC1 with intracellular protein FKBP12, inhibiting mTOR activity and subsequently reducing the activity of mTOR downstream effectors S6 ribosomal protein kinase (S6K1) and eukaryotic elongation factor 4E-binding protein (4E-BP)
Other Activities: Inhibits the expression of hypoxia-inducible factors (e.g., HIF-1) and reduces the expression of vascular endothelial growth factor (VEGF), exhibiting anti-angiogenic effects
IV. Pharmaceutical Information
Developer: Novartis (Switzerland)
Indications:
Adult patients with non-resectable, locally advanced or metastatic, well-differentiated, progressive neuroendocrine tumors of gastrointestinal or lung origin
Adult patients with advanced renal cell carcinoma who have failed prior therapy with sunitinib or sorafenib
Adult patients with unresectable, locally advanced or metastatic, well-differentiated (moderately differentiated or highly differentiated) progressive pancreatic neuroendocrine tumors
Adult and pediatric patients with tuberous sclerosis complex (TSC)-associated subependymal giant cell astrocytoma (SEGA) requiring therapeutic intervention but not suitable for surgical resection
Dosage and Administration: The recommended dosage is 10mg orally once daily, taken at the same time each day, either with or without food
Adverse Reactions: May include non-infectious pneumonia, infections, stomatitis, renal failure, etc.
Contraindications: Contraindicated in patients with hypersensitivity to any component of this product, other rapamycin derivatives, or any excipient in this product

Tai Yau Street, San Po Kong, Kowloon, Hong Kong, China.