- Synthetic anti-infective drugs
- Medications for the digestive system
- Antipyretic and analgesic drugs
- Medications for the blood system
- Medications for the respiratory system
- Anti-allergic drugs
- Medications for the urinary system
- Diagnostic medications
- Immunosuppressive and immunomodulatory drugs
- Vitamins and mineral supplements
- Antioxidants and medications for osteoporosis
- Antiparasitic drugs
- Ophthalmic medications
- Amino acids and their derivatives
- Dermatological medications
- Medications for the circulatory system
- Antitumor drugs
- Medications for the nervous system
- Hormonal and endocrine function-regulating drugs
- Antibiotics
- Others
CAS NO.: 3254-89-5
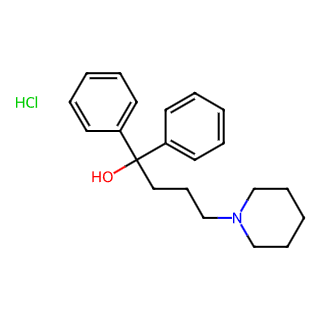



Difenidol Hydrochloride
Difenidol Hydrochloride, also known as Difenidol HCl, has the Chinese name of Dihydrochloride Difenidol or simply Dihydrochloride Diphenidol. It is a commonly used pharmaceutical compound primarily utilized for the prevention and treatment of vertigo, nausea, and vomiting caused by various reasons or diseases, particularly motion sickness during travel in vehicles, ships, airplanes, etc.
Basic Information
English Name: Difenidol Hydrochloride
Chinese Name: 盐酸地芬尼多
Alternative Names: Difenidol, Diphenidol, etc.
Molecular Formula: C21H27NO (Note: Some sources may include HCl in the formula as C21H28ClNO, accounting for the hydrochloride salt)
Molecular Weight: Approximately 309.45 (excluding HCl)
CAS Number: 3254-89-5
Pharmacological Effects
Difenidol Hydrochloride enhances the blood supply to the vertebral basilar artery, modulates the function of the vestibular system, and inhibits the vomiting center, thereby exerting antiemetic and anti-vertigo effects. It is also a non-selective muscarinic M1-M4 receptor antagonist with antiarrhythmic activity and is an effective non-specific blocker of voltage-gated ion channels (Na+, K+, Ca2+) in nerve cells.
Indications and Dosage
Indications: Used for the prevention and treatment of vertigo, nausea, and vomiting caused by various reasons or diseases, including motion sickness.
Dosage: The specific dosage should be determined by the physician or pharmacist based on the patient's condition. It is usually administered in tablet form orally.
Adverse Reactions
Common adverse reactions include dry mouth, palpitations, dizziness, headache, drowsiness, restlessness, and mild gastrointestinal discomfort, which usually disappear after discontinuation.
Rarely, severe adverse reactions such as auditory hallucinations, visual hallucinations, disorientation, mental confusion, depression, and rash may occur.
Transient hypotension reactions have also been reported.
Contraindications and Precautions
Contraindications: Prohibited for infants under 16 months old, patients with renal insufficiency, and individuals with hypersensitivity to this drug.
Precautions:
Consult a physician or pharmacist for pediatric dosage.
Use with caution in patients with glaucoma, gastrointestinal or urinary tract obstruction, and tachyarrhythmia.
Use with caution in pregnant women.
In case of overdose or severe adverse reactions, seek medical attention immediately.
Keep out of reach of children.
Children should use this medication under adult supervision.
If using other medications, consult a physician or pharmacist before taking Difenidol Hydrochloride.
Storage and Shelf Life
Storage Conditions: Generally, store in a sealed container at a temperature range of -80°C to 20°C, depending on the product form and packaging requirements.
Shelf Life: Typically 24 months, but may vary by manufacturer and product batch.
Suppliers and Specifications
Difenidol Hydrochloride is produced by various pharmaceutical manufacturers. Common specifications include tablets containing 25 mg of Difenidol Hydrochloride per tablet. Packaging varies, such as aluminum-plastic packaging with 30 tablets per box.
This translation provides a comprehensive overview of Difenidol Hydrochloride, including its basic information, pharmacological effects, indications, dosage, adverse reactions, contraindications, precautions, storage conditions, shelf life, and supplier information.

Tai Yau Street, San Po Kong, Kowloon, Hong Kong, China.