- Synthetic anti-infective drugs
- Medications for the digestive system
- Antipyretic and analgesic drugs
- Medications for the blood system
- Medications for the respiratory system
- Anti-allergic drugs
- Medications for the urinary system
- Diagnostic medications
- Immunosuppressive and immunomodulatory drugs
- Vitamins and mineral supplements
- Antioxidants and medications for osteoporosis
- Antiparasitic drugs
- Ophthalmic medications
- Amino acids and their derivatives
- Dermatological medications
- Medications for the circulatory system
- Antitumor drugs
- Medications for the nervous system
- Hormonal and endocrine function-regulating drugs
- Antibiotics
- Others
CAS Number: 179324-69-7



Basic Information
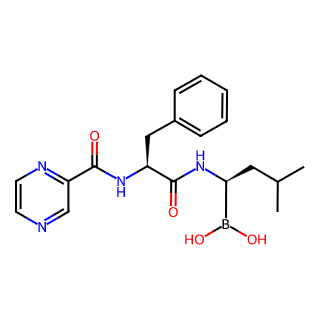
Product Name: Bortezomib
CAS Number: 179324-69-7
Molecular Formula: C19H25BN4O4
Molecular Weight: 384.23700 (alternatively, 384.24, with minor differences possibly due to measurement methods and precision)
Physical Properties
Appearance and Characteristics: Yellow solid
Melting Point: 122-124°C (alternatively, 139-143°C, which may be due to differences in batches or preparation methods)
Density: 1.214 (or 1.2±0.1 g/cm³)
Refractive Index: 1.564
Polarizability: 40.8±0.5 10^-24 cm³
Vapor Pressure: 5.1X10^-20 mm Hg at 25°C (estimated)
Solubility: Soluble in organic solvents such as DMSO (76 mg/l), but poorly soluble in water (<1 mg/ml)
Chemical Properties
Category: A dipeptide boronic acid that reversibly inhibits the 26S proteasome and is also an effective inhibitor of the 20S proteasome (20S proteasome) with a Ki of 0.6 nM
Mechanism of Action: Interferes with cancer cell growth and proliferation by inhibiting the activity of intracellular proteasomes, disrupting the cell cycle, inducing apoptosis, and inhibiting nuclear factor NF-κB
Pharmaceutical Properties
Indications:
Combined with melphalan and prednisone (MP regimen) for the treatment of previously untreated patients with multiple myeloma who are not suitable for high-dose chemotherapy and bone marrow suppression
As monotherapy for the treatment of patients with relapsed multiple myeloma who have received at least one prior therapy
For the treatment of patients with relapsed or refractory mantle cell lymphoma (safety and efficacy data for this indication are primarily from foreign clinical studies, and clinical study data for the Chinese population are lacking)
Route of Administration: For intravenous injection only; intrathecal injection can be fatal
Dosage and Administration:
When combined with melphalan and prednisone, the recommended dosage is a single injection of 1.3 mg/m², administered twice weekly for 2 weeks, followed by a 10-day rest period, with 3 weeks constituting one treatment cycle
For maintenance therapy beyond 8 cycles, the standard regimen may be followed, or a maintenance regimen of once weekly for 4 weeks, followed by a 13-day rest period, may be used
Dosage adjustments should be made based on the patient's actual condition and toxicity
Precautions:
During bortezomib treatment, patients require frequent blood tests to monitor its effects on the hematopoietic system
Patients should follow the doctor's advice, take medication according to the prescribed dosage and schedule, and pay close attention to possible adverse reactions
Store away from light and refrigerate to maintain stability

Tai Yau Street, San Po Kong, Kowloon, Hong Kong, China.