- Synthetic anti-infective drugs
- Medications for the digestive system
- Antipyretic and analgesic drugs
- Medications for the blood system
- Medications for the respiratory system
- Anti-allergic drugs
- Medications for the urinary system
- Diagnostic medications
- Immunosuppressive and immunomodulatory drugs
- Vitamins and mineral supplements
- Antioxidants and medications for osteoporosis
- Antiparasitic drugs
- Ophthalmic medications
- Amino acids and their derivatives
- Dermatological medications
- Medications for the circulatory system
- Antitumor drugs
- Medications for the nervous system
- Hormonal and endocrine function-regulating drugs
- Antibiotics
- Others
CAS Number: 86329-79-5



I. Basic Information
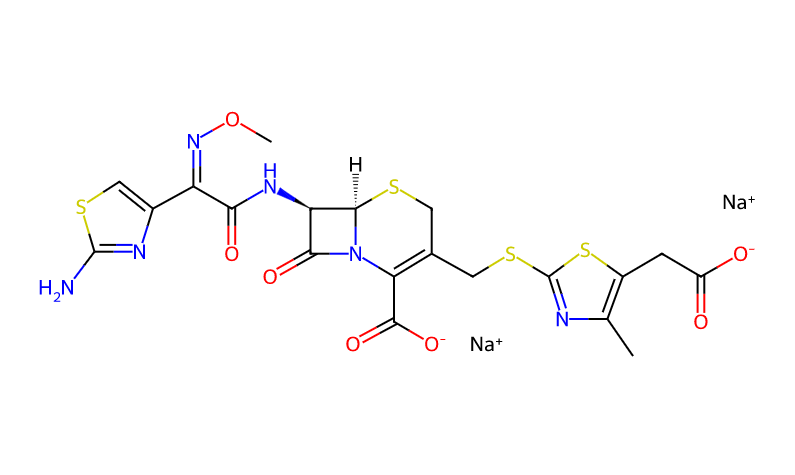
Product Name: Cefodizime Sodium
CAS Number: 86329-79-5
Molecular Formula: C20H21N6NaO7S4
Molecular Weight: 608.65
II. Physical Properties
Appearance: Cefodizime Sodium is a white to slightly yellow powder or crystalline powder, odorless or with a slight specific odor.
Solubility: It is highly soluble in water but almost insoluble in anhydrous ethanol or ether.
Melting Point: 196~207°C
III. Chemical Properties
Stability: It has a certain degree of hygroscopicity and should be stored at 2~8°C.
Reactive Characteristics: Cefodizime Sodium is a third-generation cephalosporin for parenteral use. It has a high affinity for proteins involved in cell wall synthesis in sensitive bacteria. It is insensitive to most β-lactamases and has antibacterial activity against both Gram-positive and Gram-negative bacteria. It is stable to β-lactamases, cephalosporinases, and penicillinases.
IV. Pharmaceutical Properties
Antibacterial Spectrum: Cefodizime Sodium has a broad antibacterial spectrum, including most clinically relevant Gram-positive, Gram-negative, aerobic, and anaerobic bacteria.
Immunoenhancing Effects: Cefodizime Sodium, invented by Hoechst AG, is the first third-generation cephalosporin with immunoenhancing properties. It enhances macrophage function, natural killer cell function, and B lymphocyte responsiveness. It also induces the production of interleukins and interferons, thereby enhancing the immune function of the human body.
Pharmacokinetics: After entering the body, Cefodizime Sodium is rapidly distributed into body fluids and tissues, with increased local concentrations exceeding the effective concentrations required to inhibit most pathogens. Most of it is excreted in non-metabolized form via the kidneys, with a serum half-life of 2.5 hours in individuals with normal renal function.

Tai Yau Street, San Po Kong, Kowloon, Hong Kong, China.