- Synthetic anti-infective drugs
- Medications for the digestive system
- Antipyretic and analgesic drugs
- Medications for the blood system
- Medications for the respiratory system
- Anti-allergic drugs
- Medications for the urinary system
- Diagnostic medications
- Immunosuppressive and immunomodulatory drugs
- Vitamins and mineral supplements
- Antioxidants and medications for osteoporosis
- Antiparasitic drugs
- Ophthalmic medications
- Amino acids and their derivatives
- Dermatological medications
- Medications for the circulatory system
- Antitumor drugs
- Medications for the nervous system
- Hormonal and endocrine function-regulating drugs
- Antibiotics
- Others
CAS NO.: 444731-52-6
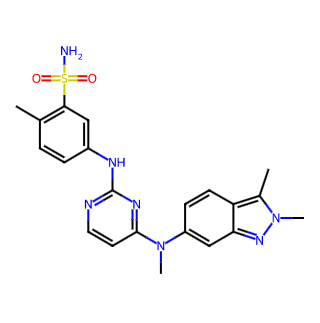



Basic Information
Chinese Name: 帕唑帕尼
English Name: Pazopanib
CAS Number: 444731-52-6
Molecular Formula: C21H23N7O2S
Molecular Weight: 437.52 (or 437.5180, may vary slightly depending on sources)
EINECS Number: 251-228-4
Mol File: 444731-52-6.mol
Physical Properties
Melting Point: 285-289°C (or 281-291°C, may vary slightly depending on sources)
Boiling Point: 728.8±70.0°C (predicted)
Density: 1.40
Flash Point: 359°C
Solubility: Soluble in acidic aqueous solutions (slightly), DMSO (slightly, with heating), methanol (slightly, with heating)
Uses and Synthesis
Uses: Pazopanib, developed by GlaxoSmithKline, is a novel oral angiogenesis inhibitor that interferes with the formation of new blood vessels essential for the survival and growth of stubborn tumors. It targets vascular endothelial growth factor receptors (VEGFRs) and works by inhibiting the formation of new blood vessels that supply tumors. It is indicated for the treatment of advanced renal cell carcinoma, soft tissue sarcoma, epithelial ovarian cancer, and non-small cell lung cancer. Clinical studies have also shown its effectiveness in treating thyroid cancer.
Synthesis Method: The synthesis route of pazopanib may involve multiple reaction steps, and the specific details may vary depending on different laboratories or production processes.
Safety Information
Risk Phrases: R43, R62/63
Safety Phrases: S36/37/39, S28B
Storage Conditions: Should be stored under refrigerated conditions at a low temperature, with the specific temperature varying depending on different suppliers or product specifications.
Clinical Applications
FDA Approval: Pazopanib has been approved by the U.S. Food and Drug Administration (FDA) for the first-line treatment of advanced renal cell carcinoma and for the treatment of patients with advanced soft tissue sarcoma who have received prior chemotherapy.
Clinical Trials: Multiple clinical trials have demonstrated the efficacy and safety of pazopanib in the treatment of advanced renal cell carcinoma, soft tissue sarcoma, and other tumors.

Tai Yau Street, San Po Kong, Kowloon, Hong Kong, China.