- Synthetic anti-infective drugs
- Medications for the digestive system
- Antipyretic and analgesic drugs
- Medications for the blood system
- Medications for the respiratory system
- Anti-allergic drugs
- Medications for the urinary system
- Diagnostic medications
- Immunosuppressive and immunomodulatory drugs
- Vitamins and mineral supplements
- Antioxidants and medications for osteoporosis
- Antiparasitic drugs
- Ophthalmic medications
- Amino acids and their derivatives
- Dermatological medications
- Medications for the circulatory system
- Antitumor drugs
- Medications for the nervous system
- Hormonal and endocrine function-regulating drugs
- Antibiotics
- Others
CAS NO.: 87333-19-5
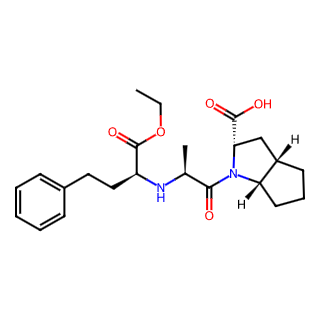



Ramipril
Basic Information
CAS Number: 87333-19-5
Chemical Name: Ramipril
Molecular Formula: C23H32N2O5
Molecular Weight: 416.52 g/mol
Physical and Chemical Properties
Melting Point: 106-108°C (alternatively reported as 107.0-110.0°C)
Density: 1.2±0.1 g/cm³
Boiling Point: 616.2±55.0 °C at 760 mmHg
Flash Point: 326.4±31.5 °C
Vapor Pressure: 0.0±1.9 mmHg at 25°C
Refractive Index: 1.556
Safety Information
Personal Protective Equipment (PPE): Recommended PPE includes protective eyewear (e.g., meeting NIOSH or EN 166 standards), gloves (conforming to EU 89/686/EEC regulations and EN 376 standards), and respiratory filters of N95 (US) or P1 (EN143) rating.
Hazard Codes (Europe): Xi
Risk Statements (Europe): R36/38 (Irritating to eyes and skin)
Safety Statements (Europe): S26 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice); S37/39 (Wear suitable gloves and eye/face protection)
Product Applications
Ramipril is primarily used for the treatment of hypertension. It is also indicated for the management of congestive heart failure and acute myocardial infarction (MI) with left ventricular dysfunction occurring within the first few days after the acute MI. The drug can be taken with or without food, and the daily dose can be administered in a single dose or divided into two doses (morning and evening) depending on the indication and dosing requirements. The addition of diuretics may enhance the antihypertensive effect of Ramipril.
Synthetic Route
The synthesis of Ramipril involves multiple steps, including esterification, acylation, hydrogenation, and other reactions, ultimately yielding the desired Ramipril crystal with specific configuration and biological activity.
In summary, Ramipril (CAS 87333-19-5) is a crucial antihypertensive medication with well-defined physicochemical properties and extensive clinical applications.

Tai Yau Street, San Po Kong, Kowloon, Hong Kong, China.