- Synthetic anti-infective drugs
- Medications for the digestive system
- Antipyretic and analgesic drugs
- Medications for the blood system
- Medications for the respiratory system
- Anti-allergic drugs
- Medications for the urinary system
- Diagnostic medications
- Immunosuppressive and immunomodulatory drugs
- Vitamins and mineral supplements
- Antioxidants and medications for osteoporosis
- Antiparasitic drugs
- Ophthalmic medications
- Amino acids and their derivatives
- Dermatological medications
- Medications for the circulatory system
- Antitumor drugs
- Medications for the nervous system
- Hormonal and endocrine function-regulating drugs
- Antibiotics
- Others
CAS No.: 397864-44-7
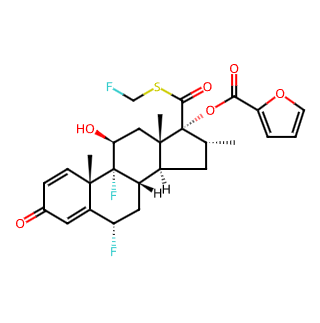



Fluticasone Furoate
Fluticasone Furoate, also known as Furoate of Fluticasone, is a topical, intranasal, synthetic trifluorinated corticosteroid with enhanced affinity.
I. Basic Properties
Chemical Structure: As a synthetic trifluorinated corticosteroid, Fluticasone Furoate possesses a specific chemical structure with a CAS number of 397864-44-7 and a molecular weight of 538.58.
Physical State: Fluticasone Furoate exists as a solid, with a white to off-white color.
Solubility: It has good solubility in solvents such as DMSO, facilitating the preparation of required concentrations for laboratory or clinical applications.
II. Pharmacological Effects
Anti-inflammatory and Anti-asthmatic Activity: Fluticasone Furoate exhibits significant anti-inflammatory and anti-asthmatic activities, effectively reducing airway inflammation and thereby alleviating symptoms of asthma and allergic rhinitis.
Low Systemic Exposure: Due to its local mode of administration, Fluticasone Furoate has low systemic exposure, reducing the risk of systemic side effects.
Inhibition of Tumor Necrosis Factor: Studies have shown that Fluticasone Furoate possesses potent efficacy in inhibiting the synthesis and action of tumor necrosis factor, further contributing to the reduction of inflammatory responses.
III. Clinical Applications
Allergic Rhinitis: Fluticasone Furoate is used in the research and treatment of allergic rhinitis. It is available as a nasal spray, formulated as an aqueous suspension of micronized Fluticasone Furoate, administered locally to the nasal mucosa via a metered dose mist spray pump.
Chronic Obstructive Pulmonary Disease (COPD): While Fluticasone Furoate is primarily associated with the treatment of allergic rhinitis when mentioned alone, it can also be used in the treatment of COPD when combined with other medications such as vilanterol. This combination therapy leverages the dual effects of an inhaled corticosteroid (e.g., Fluticasone Furoate) and a long-acting β2-agonist (e.g., vilanterol) to improve lung function and reduce COPD exacerbations.

Tai Yau Street, San Po Kong, Kowloon, Hong Kong, China.