- Synthetic anti-infective drugs
- Medications for the digestive system
- Antipyretic and analgesic drugs
- Medications for the blood system
- Medications for the respiratory system
- Anti-allergic drugs
- Medications for the urinary system
- Diagnostic medications
- Immunosuppressive and immunomodulatory drugs
- Vitamins and mineral supplements
- Antioxidants and medications for osteoporosis
- Antiparasitic drugs
- Ophthalmic medications
- Amino acids and their derivatives
- Dermatological medications
- Medications for the circulatory system
- Antitumor drugs
- Medications for the nervous system
- Hormonal and endocrine function-regulating drugs
- Antibiotics
- Others
CAS NO.: 9041-08-1



Basic Information
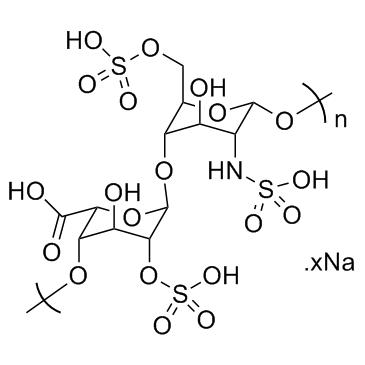
Chinese Name: 肝素钠
English Name: Heparin sodium
CAS Number: 9041-08-1
Molecular Formula: (C12H16NS2Na3)20 (Note: This represents the polymeric form of heparin sodium, and the actual molecular structure is more complex.)
Molecular Weight: Ranging from 3,000 to 30,000 Daltons, with an average molecular weight of approximately 15,000 Daltons
Physical Properties
Solubility: Easily soluble in water, insoluble in organic solvents such as ethanol, ether, and acetone
Melting Point: >181°C (decomposes)
Specific Rotation: [α]25D +47° (c=1.5, in water)
pH Value: 6.0–8.0 (10g/l, 25°C)
Chemical Properties
Heparin sodium is an acidic mucopolysaccharide containing sulfate groups, which is a natural anticoagulant. It has a strong negative charge and can form molecular complexes with some cations.
The anticoagulant effect of heparin sodium is closely related to the sulfate groups in its molecule. Destruction of these sulfate groups can reduce its anticoagulant activity.
Uses
Medical Uses: Primarily used in the treatment of acute thromboembolic diseases, disseminated intravascular coagulation (DIC), etc. It prevents platelet aggregation and destruction, inhibits the conversion of fibrinogen to fibrin monomers, inhibits the formation of thrombinogen, and counteracts the formed thrombinogen, among other effects.
Biochemical Research: Also used in biochemical research, such as preventing the conversion of prothrombin to thrombin.
Safety Information
Hazard Description: Overdose of heparin sodium can lead to dangerous conditions such as spontaneous bleeding, necessitating strict control of dosage.
Storage Conditions: Should be stored in a cool, dry, and light-protected place, typically at a recommended storage temperature of 2-8°C.

Tai Yau Street, San Po Kong, Kowloon, Hong Kong, China.